Integrated Nanosystems Research Facility
Newsletter Issue 10
September 28 | Issue 10
Welcome to the new INRF monthly newsletter where we will be sharing equipment capabilities, updates and research accomplishments.
Faculty Spotlight – Abraham ‘Abe’ Lee, Ph.D.

The month, we present a longtime member of the INRF research community, Abraham “Abe” Lee, Ph.D. Lee is professor of biomedical engineering (BME) and mechanical and aerospace engineering (MAE) at UC Irvine. He is director of the NSF I/UCRC Center for Advanced Design & Manufacturing of Integrated Microfluidics (CADMIM). Lee served as editor-in-chief for the Lab on a Chip journal from 2017 to 2020. Prior to UCI, he was at the National Cancer Institute and was a program manager in the Microsystems Technology Office at DARPA (1999-2001), senior technology advisor at National Cancer Institute (NCI) and a group leader with Lawrence Livermore National Lab (LLNL). Over the years, Lee has pioneered research in applying microfluidics to biomedical applications, and he currently focuses on integrated microfluidic systems for precision medicine including liquid biopsy, microphysiological systems, cell engineering and immunotherapy. His research has contributed to the founding of several startup companies. He owns 55 issued U.S. patents and is author of over 130 journals articles. Lee was awarded the 2009 Pioneers of Miniaturization Prize and is an elected fellow of the National Academy of Inventors (NAI), the American Institute of Medical and Biological Engineering (AIMBE), the Royal Society of Chemistry (RSC), the American Society of Mechanical Engineering (ASME) and the Biomedical Engineering Society (BMES).
Research Theme: microfluidic engineering for vein-to-vein therapies
Precision medicine is the paradigm of developing treatments for patients based on molecular and cellular targets that are effective in vivo when administered. That is, one must not only be able to identify molecular and cellular targets that are the source of disease, but also understand how these targets behave in the body based on physiological principles. Microfluidics bridges the scales of molecular, cellular tissue, and can even recapitulate organ-level microphysiological functions of the body, essentially enabling microprocessors for a plethora of health indicators. Recent developments in microfluidics have contributed to burgeoning precision medicine fields such as liquid biopsy, immunotherapy, single cell analysis, genotyping and gene sequencing, and microphysiological systems. Research projects in Lee’s lab will be working toward the vision of closing the loop from patient sample to personalized medicine from harvesting specific cell populations from blood (e.g. immune cells and rare cells) followed by the development of patient-specific therapies (including but not limited to cell therapy, immunotherapy, targeted therapy). As such, the vision is for microfluidic technologies to close the loop from detection to diagnosis, to therapy.
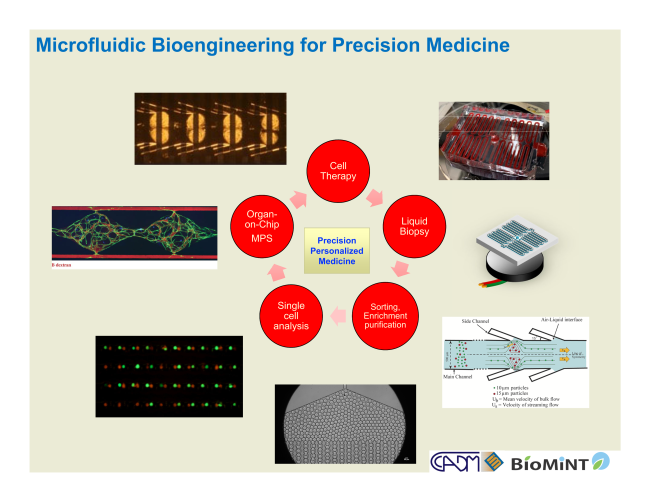
Immediate Research Projects:
Acoustic microvortices instrumentation for dosage controlled, high efficiency cell engineering for universal CAR-T cells: Lee’s lab has developed the Acoustic-Electric Shear Orbiting Poration (AESOP) platform that addresses several of the known challenges for nonviral transfection techniques, including cell viability and dosage-controlled intracellular delivery while achieving relatively high throughput. AESOP is based on a microfluidic platform termed “lateral cavity acoustic transducers (LCATs)” that can form an array of microvortices activated by acoustic energy. By trapping cells to tumble in whirlpool-like microvortices, this platform optimizes the delivery of intended cargo sizes with uniform poration of the cell membranes via mechanical shear followed by the modulated enlargement of these nanopores via electric field. Using AESOP, we will focus on technology development for producing universal CAR T cells. By controlling dosage, a decreased amount of CRISPR reagents in cells could reduce off-target effects. Furthermore, the uniform delivery enables the delivery of large cargo sizes necessary for immunotherapy-related gene transfection and gene editing. This is the basis of an NIH R01 project with four specific aims: 1- Generate acoustic microstreaming vortices for uniform cell membrane nanopores and uniform local mixing; 2- Demonstrate uniform electric field enlargement of nanopores for cargo delivery; 3- Design prototype AESOP instrumentation and 4- Quantitative benchmark of AESOP for intracellular delivery of large size cargos with dosage control for the development of universal CAR T cells.
Microfluidic Precision Engineered Artificial Antigen Presenting Cells for Cancer Immunotherapy: Another platform that Lee’s lab has developed is the microfluidic process to generate cell-sized unilamellar vesicles (CUVs) that are decorated with antigen presenting ligands for artificial APCs (or aAPCs). Preliminary results show that aAPCs are able to bind and interact with T cells and cause their expansion. Their R33 (pending) project aims to further optimize the aAPCs preparation and test its capacity to induce tumor specific responses in vitro and in vivo. The hypothesis is that the optimized aAPC functionalization will result in enhanced expansion of cytotoxic CD8 T cells and a reduction in tumor progression over the present one (original). The Specific Aims are- 1) Bioinspired optimization of artificial antigen presenting cell (aAPC) production via microfluidic engineering. 2) Evaluation of the capacity of aAPCs to induce tumor specific T cell responses in vitro. 3) Evaluation of the capacity of aAPCs to induce T cell responses and tumor killing with an in vivo mice tumor model. Methods to scale up the production of aAPCs for in vivo use will be developed. The goal is to produce an aAPC preparation that mimics cells, is stable, easy to produce in large quantities and capable of expanding tumor specific CD8 T cells for immunotherapy of cancer.
Microfluidic liquid biopsy and vascularized microphysiological systems (VMPS): Collaborating with an oncologist they have developed a microfluidic platform that can separate and enrich the different cell populations in whole blood for liquid biopsy and immunotherapy applications. Using this platform, they studied the CTCs (circulting tumor cells) and companion circulating cancer-associated fibroblasts (cCAFs) as well as the state of the lymphocytes of the cancer patients that are either locoregional or metastatic and found a better correlation of CTCs and cCAFs with metastatic stage 4 patients. Along with collaborators, they have developed a vascularized microphysiological system that has perfused blood vessels in 3D tissues. This platform has been commercialized and is contributing to the drug development process including drug screening, toxicity studies, and immuno-oncology. In Lee’s future research, he intends to combine personalized VMPS and liquid biopsy with biotherapeutics that include cell therapy, targeted drug delivery, and immunotherapy.

Equipment Spotlight
HEX 03 030205 Hot Embossing System
The HEX03 is a high precision hot embossing system. It is designed to allow embossing and thermal micro-molding of planar microstructures in thermoplastic workpiece materials (Polymers). Typical applications are microfluidic channel system, integrated optical, diffractive-optical and micro-optical components.

Specifications:
- Embossing Force: Up to 200KN
- Substrate holder size: 180mm
- Motion speed: 1um/min -10mm/S
- Substrate thickness: 10mm
- Demolding pressure :up to 90 Psi
- Max temperature: 220 ﹾC
- Heating plate: Upper and Lower
- Variability of chamber height: 10 mm
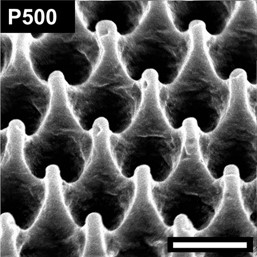
Surface:P500 Period (nm):500 Width (nm):100
Height (nm) 700
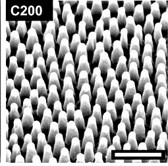
Surface:C200 Period (nm):170 Width (nm):70
Height (nm) 210

Before Imprinting

After Imprinting



